Thermoplastic materials
Thermoplastic is a pliable polymer material that becomes flexible or moldable. The higher specific temperature helps in the formation of thermoplastic materials. Thermoplastic materials harden upon chilling. These materials have higher molecular mass. Some polymer chains are connected through intermolecular interactions. They deteriorate quickly with higher temperatures. It yields a gelatinous fluid. We can reshape thermoplastic materials. We use it characteristically to harvest portions. We can use numerous polymer dispensation methods such as
- Injection molding
- Compression molding
- Calendaring
- Extrusion

Thermoplastics and thermosetting materials are completely different. These thermoset materials procedure irretrievable chemical bonds. We can observe these bonds during the curing process. Manufacturers observe no melting of thermosets. They show melting when there is a higher temperature. They characteristically decay. They do not reform when the temperature is low.
Stress-strain graph of a thermoplastic material
Over the glass changeover temperature, there is a change in thermoplastic material. Thermoplastic material under its melting point shows change. There are physical characteristics of a thermoplastic modification. The change is tremendous without any related phase alteration. Roughly they do not completely crystallize. No gel formation during glass conversion temperature. They maintain some of their nebulous features.
Types of Plastic
- Amorphous plastic
- Semi-amorphous plastic
These plastic materials are important when high optical lucidity is compulsory. In this process, light scatters powerfully by crystal-type materials. Their wavelength is larger. These Nebulous and semi-amorphous materials are less resilient. They show less hindrance to chemical attacks. Ecological strain results in cracks. These materials dearth a crystalline construction.
Acrylonitrile butadiene styrene
Acrylonitrile butadiene styrene is a special polymer. It forms by a combination of
- Styrene
- Acrylonitrile
- Polybutadiene
It is an insubstantial material. It shows high-impact confrontation. It has power-driven durability. It postures insufficient perils to human wellbeing. It may cause different threats under regular treatment. We can use it in several usable products. Manufacturers use them in models, appliances, and phones.
We can reduce brittleness with the accumulation of plasticizers. We can increase the flexibility of unstructured chain sections. These help in lowering the glass transition temperature. We can adjust the polymer through copolymerization. Totaling the chain reactions that don’t react to monomers. Lowering can happen before polymerization. These procedures are helpful in pliable automobile fragments. They are rectilinear or somewhat divided long-chain particles. They are capable of frequently relaxing on heating. They become hard when cooled.
Nylon
Polyamides is a class and Nylon is its major component. It has assisted as an auxiliary component.
- Hemp
- Cotton
- Silk
Nylon fibers are advantageous in the construction of textiles, cables, mats, and musical threads. We use unpackaged nylon for power-driven portions. It includes engine rivets, types of machinery, and power utensil coverings. We can use it in the production of heat-resistant merged constituents.
Polyether sulfone or Polysulfone
Polyether sulfone is a type of particularly planned thermoplastics. Higher thermal stability, higher oxidative stability, and higher hydrolytic stability. They have good reactance to the following materials, aqueous inorganic acids, bases, salt solutions, lubricants, and blubbers.
Polyoxymethylene
POM is a polyformaldehyde. We can call it acetal. It is helpful in the formation of thermoplastic materials. We can use it in exactness shares. They require high toughness. It provides low friction. It also provides exceptional dimensional steadiness. It produces different chemicals. It forms unique formulas to some extent. Their names are Delrin, Duracon, Celcon, and Ramtal.
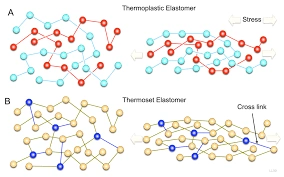
A thermosetting polymer is often stated too as thermosetting. We can obtain a polymer by irreversible curing. It is a process in which we cure the soft solid or viscous liquid. We include curing by heating. We can use appropriate radiation in its formation. We can promote it by high pressure. It can also form its shape by mixing with a catalyst. The heat does not essentially radiate from the outside.
Curing
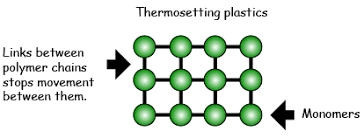
Manufacturers frequently generate it by the reaction of the resin with the catalyst. “Curing” results in chemical reactions. These Chemical reactions create widespread cross-linking. These links form between the chains of polymers. They form a well-shaped and unsolvable polymer system.
Properties
The starting material for thermoforming is malleable. It is a liquid before hardening. It is often intended to form the ultimate shape. We can use it as a paste. When we cure the material, it is not possible for us to melt it for reformulation. Unlike thermoplastic polymers, which we typically produce and supply as tablets.
Manufacturers mold them into a specific figure of the ultimate product. We can also reshape it by melting, grinding, inserting pressure, or inoculation molding.
By creating covalent connections between the polymer’s constituent chains, we can attain a specific shape. Crosslinking or chain extension occurs during the curing process. Turning a thermosetting mastic into a rubber or elastomer. The density of thermoset materials varies according to the polymers in the mixture. It depends on the monomer or prepolymer mix. It also depends upon the crosslinking procedure.
Unsaturated polymers
Unsaturated sites on the backbone or at the ends of acrylic resins play an important role. We can link polyesters and vinyl esters. These reactions are a reason for the link between copolymers. Manufacturers initiate the process of “Curing” process from unsaturated monomer diluents. Free radicals’ production is associated with it. Ionizing radiation plays a role in photolytic or updraft decay. The radical initiator has a specific intensity for cross-linking. We can influence it by the step of unsaturation in the polymer.
Strength
We can copolymerize epoxy-efficient gums by nucleophilic addition reactions. Some processes like cationic or anionic catalytic processes have their own importance in these reactions. We can homopolymerize it by using these reagents and heat.
“Thermosetting plastics are mostly stronger than thermoplastic materials”
Thermosetting plastics are stronger due to the three-dimensional network. Cross bonding or crosslinking is also better in working with high-temperature. They have wide applications up to the breakdown temperature. They have the ability to keep their shape as robust covalent bonds. We cannot break the bonds between polymer chains easily. Their bond is directly proportional to their shape.
